Tick-Borne Encephalitis Virus Nonstructural Protein 1 IgG Enzyme-Linked Immunosorbent Assay for Differentiating Infection versus Vaccination Antibody Responses (Poster Abstract)
Philipp Girl a, Malena Bestehorn-Willmann d, Sabine Zangea, Johannes Borde b, c, Gerhard Doblera,
Heiner von Buttlara
a Bundeswehr Institute of Microbiology, Munich, Germany
b University of Freiburg Medical Center and Faculty of Medicine, Freiburg, Germany
c Praxis Dr. J. Borde/Gesundheitszentrum Oberkirch, Oberkirch, Germany
d Institute of Zoology, Parasitology, University of Hohenheim, Stuttgart, Germany
Introduction
Tick-borne encephalitis (TBE) is the most important vector transmitted viral infection in Europe. The disease is caused by the tick-borne encephalitis virus (TBEV), a member of the Flaviviridae family. For TBE no causal treatment is known but the available inactivated vaccines provide good protection. TBE-diagnosis is mainly based on the serological detection of specific antibodies against TBEV. So far , the core challenges in TBE-diagnosis are the lack of distinguishability between vaccination induced and infection induced antibodies and the strong cross-reactivity with other flaviviral infections or vaccinations. In this study a new TBEV antibody ELISA based on the non-structural protein 1 (NS1) was presented. The new ELISA was validated for use in accredited diagnostic laboratories according to the European norm DIN EN ISO 15189. Sensitivity and specificity were determined, in particular regarding to the differentiation between vaccination induced and infection induced antibodies as well as the robustness against potentially cross-reacting patient’s sera.
Materials and Methods
Samples were received from TBE patients, patients with other confirmed disease (including other flavivirus infections), TBE vaccine recipients and patients vaccinated against other flaviviruses (YFV, JEV). To avoid false positive results, during test establishment a cut-off control (YFC) was introduced before starting the sensitivity and specificity studies.
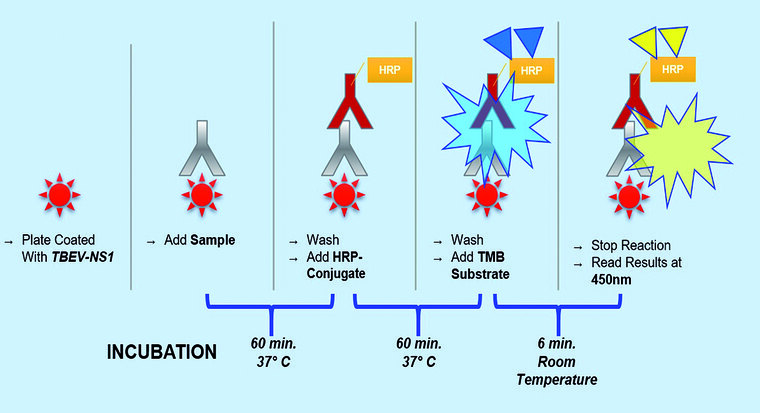
Figure1: Illustration of the procedure.
YFC is the serum of a person, vaccinated against TBEV and YF, that showed the highest OD value of all YF vaccinees used in the pre -testing development. The YFC was run in triplicates in each ELISA run to determine the threshold. The mean YFC OD plus one standard deviation was defined as the negative threshold . A YFC OD of higher than three standard deviations of YFC mean value was set as the positive threshold. Samples with an OD higher than the positive threshold of the mean YFC OD were considered positive. Samples with an OD lower than the YFC negative threshold OD were validated as negative. Samples with an OD in between mean YFC negative and positive threshold were considered borderline.
Results
Among the 71 patients acutely ill with TBE, 67 were tested positive for NS1 specific IgG antibodies, resulting in a sensitivity of 94.37 %. All but one TBE-seronegative patient tested negative in the NS1-ELISA (specificity 97.06 %). Furthermore, 49 TBE-vaccinees with verified IgG antibody titres were tested, all showed no reactivity in the NS1 IgG ELISA (specificity 100 %). To evaluate possible cross-reactions with other flaviviruses, 9 sera from patients acutely ill with DENV infection, one patient acutely ill with WNV infection, one JE-vaccinee, and 19 YF-vaccines were tested. With one exception, all DENV, WNV and JE positive samples tested negative for NS1 specific IgG antibodies, indicating a specificity of 90.91 %. Slight cross-reactivity occurred in YF vaccinated patients. Four of the 19 tested sera showed weak reactivity in the TBEV NS1 ELISA. Thus, specificity within this group was only 73.68 %. The overall specificity across all tested specimens was 93.81 %, accuracy was 94.02 %, the positive and negative predictive values (PPV/NPV) were 90.54 % and 96.36 %, respectively.
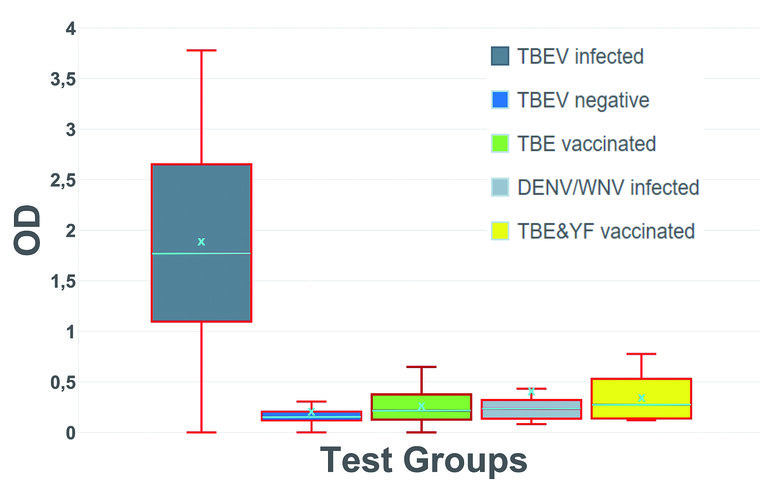
Figure 2: Optical density (OD) distribution within the tested serum groups; Group 1: IFFA confirmed TBEV infection (n = 71); Group 2: IFFA confirmed TBEV seronegative (n = 34); Group 3: TBE vaccinated (n = 49); Group 4: IFFA confirmed DENV/WNV infection (n = 10); Group 5: YF/YF and TBE/JE vaccination (n = 20); turquoise color: X = mean value; center line = median value
Conclusions
- It could be shown that the new NS1-ELISA enables a reliable differentiation between antibodies fallowing TBE-vaccination and antibodies fallowing TBEV-infection.
- The assay is furthermore much more resistant against cross-reactions.
- Thereby, the new ELISA provides high sensitivity along with high specificity.
- This new diagnostic tool is outstanding and suitable for the differentiation of questionable cases of vaccination breakthrough infections/vaccination failure as well as the examination of patients with contact to other flavivirus infections or flavivirus vaccinations.
- Additionally, for the first time since the introduction of the TBE-vaccination, working on epidemiological issues based on serological data will be possible with the new NS1-ELISA again.
For the authors
Major Dr. Philipp Girl, Veterinarian
Bundeswehr Institute of Microbiology
Neuherbergstrasse 11, D-80937 Munich
E-Mail: philippgirl@bundeswehr.org
Das Poster wurde mit dem 2. Preis des Posterwettbewerbs beim 51. Jahreskongress der Deutschen Gesellschaft für Wehrmedizin und Wehrpharmazie e. V. in Rostock-Warnemünde (23.-24. Oktober 2020) ausgezeichnet.